Guidance for Enterprises on Importing Epidemic Prevention Supply
2020-03-06
A novel coronavirus epidemic (COVID-19) broke out at the beginning of 2020. Facemasks, goggles and protective clothing soon became short in supply due to the unexpectedly rapid spread of the virus. Currently, enterprises from both within and outside of the medical supply sector are working hard to meet the massive demand on the market. Unfortunately, it simply is not enough at the moment as more people are returning to work, exponentially increasing the demand. In light of the supply shortage in China, many companies are also starting to opt for import as an alternative.
However, according to the United Nations Merchandise Trade Statistics (2018), confirmed COVID-19 cases were also reported in most of the top 10 countries that China imports surgical masks from. As a result, demand for supplies in these countries are also growing immensely, leading to export restrictions. Aside from that, their production power is also much weaker compared to China, meaning there was not ample supply to begin with. Therefore, importing from those countries can be extremely difficult considering the aforementioned issues.
Epidemic prevention supplies currently in need to be imported can be classified into several categories: donation, self-use, direct sales, and raw materials required to manufacture more supplies. In order for enterprises to import in an efficient and safe manner, it is advised to understand not only the scope and nature of epidemic prevention supplies, import regulations, import process and available favorable policies, but also the potential legal risks when entering into and performing an import contract.
Due to limited length, this article focuses on analyzing matters in relation to enterprises in need of epidemic prevention supplies for self-use, business operation and production. Duty-free importation for donation and postal import (express parcels, postal parcel and hand-carrying) will not be discussed herein.
I.Scope and Nature of Epidemic Prevention Supply
1.Scope of Epidemic Prevention Supply
Epidemic prevention supply is not a legal term. It refers to supplies needed in the prevention, containment and treatment of the epidemic (e.g. therapeutic drugs, disinfection items, protective items and medical devices).
Enterprises in need should be able to distinguish whether its imported epidemic prevention supplies are considered as general goods or medical devices, in order to follow the correct customs clearance protocols.
2.Definition and Regulations of Medical Device
According to the Regulations for the Supervision and Administration of Medical Devices, the term “medical device” refers to devices, equipment, instruments, vitro diagnostic reagents and calibrators, materials, and other relevant items, such as needed computer software.
Following the Regulations for the Supervision and Administration of Medical Devices, the Provisions for the Supervision and Management of Medical Device Operation and the Provisions for the Inspection, Supervision and Administration of Imported Medical Devices, the production, operation and importation of medical devices are subject to classified management:
Class I
refers to medical devices with low risks. Its administration and production qualification are subject to filing only, and operation does not require a license or filing. Epidemic prevention supplies in this category include: isolation gowns and caps, protective gloves (non-sterile), isolation shoe covers (non-sterile), isolation shields (non-sterile), surgical gowns and caps (non-sterile).
Class II
refers to medical devices with moderate risks. Its administration and production qualification are subject to registration and require a Medical Device Production License as well as a corresponding Registration Certificate for Medical Device, and operation is subject to filing. Such epidemic prevention supplies include: protective masks, protective suits, protective gloves (sterile), surgical masks, surgical gowns and caps (sterile), and ultraviolet disinfectors.
Class III
refers to medical device with relatively high risks. Its administration and production qualification are subject to registration and operation subject to licensing, requiring a Permit for Medical Device Operation. Such epidemic prevention supplies include care products for contact lenses.
Imported medical devices shall be registered and filed following relevant regulations in China, and obtain the Registration Certificate for Imported Medical Device, or the Filing Receipt of Imported Medical Device. Some customs are offering facilitated clearance for enterprises to import medical devices without pre-existing registration or filing in China (for self-use, donation, or government procurement). Swift customs clearance is based on the strength of relevant proof, with no registration or filing documents required.
It is advised for enterprises in need of epidemic prevention supplies and raw materials for self-use, production, and sales to check the official website of State Drug Administration to confirm whether the supplies in question are deemed as medical devices. If so, it has to be further confirmed whether or not it has already been registered/filed in China, or if it meets the conditions for exemption.
II.Qualification Requirements on Epidemic Prevention Supply Importers
1.Epidemic Prevention Supply Importer's Qualification
a.Non-Medical Device Import
Non-medical device epidemic prevention supplies are imported as general goods. Importers have to acquire the import-export management rights or seek a qualified foreign trade agency to handle the import.
b.Medical Device Import
The Provisions for the Inspection, Supervision and Administration of Imported Medical Devices provides that imported medical devices are classified into three different risk groups: moderate risks, relatively high risks, and high risk. Importers are classified as class I, II and III. The process of inspection and administration for said devices of different risk groups varies based on the classification of the importers themselves.
Enterprises planning to import medical devices as epidemic prevention supplies must complete relevant filing or obtain necessary licensing for medical device production or operation based on the classification and risk level of the medical device. Unqualified enterprises who wish to import should seek medical device importers with the right qualification to handle the procedure.
2.Review and Approval Procedure for Medical Device Under Emergency Situation
According to the Review and Approval Procedure for Medical Device Registration Under Emergency Situation (Device (2009) No. 565 of NFDA) and the approval procedures published by provincial drug administration, during the epidemic, all applicable items for emergency application shall be handled immediately upon receipt. Provided that the quality of the drug and/or medical device in question can be guaranteed by the applicants, the review and approval process shall be sped up following regulations currently in effect, whereas the verification process (e.g. technical review and evaluation, on-site inspection, and examination) can be conducted retroactively.
Eligible companies that are not yet qualified manufacturers or operators of medical devices, such as companies crossing over from other sectors to produce medical devices, can apply for emergency review and approval following the regulations of the local drug administrations so that all required documents will be present for importation.
III.Customs Clearance for Epidemic Prevention Supplies
Starting from January 25, 2020, emergency announcements have been made by local authorities across China to ensure swift customs clearance for COVID-19 epidemic prevention supplies.
For instance, the provincial government of Guangdong issued Policies and Measures on Addressing COVID-19 Epidemic and Supporting Enterprises to Resume Work and Production, proposing to open an “express path” for epidemic prevention supplies with the proper documentations so they could skip certain steps in the clearance process. In addition, measures such as “two-step declaration”, “declaration in advance”, and “guaranteed release” shall be applied to epidemic prevention supplies to ensure zero latency at customs clearance.
Meanwhile, customs in other parts of China are making exceptions for enterprises without import-export management rights to make self-declarations whenever necessary for relevant medical supplies. Enterprises are advised to consult their local authorities for details.
IV.Policy facility for Epidemic Prevention Supply Import
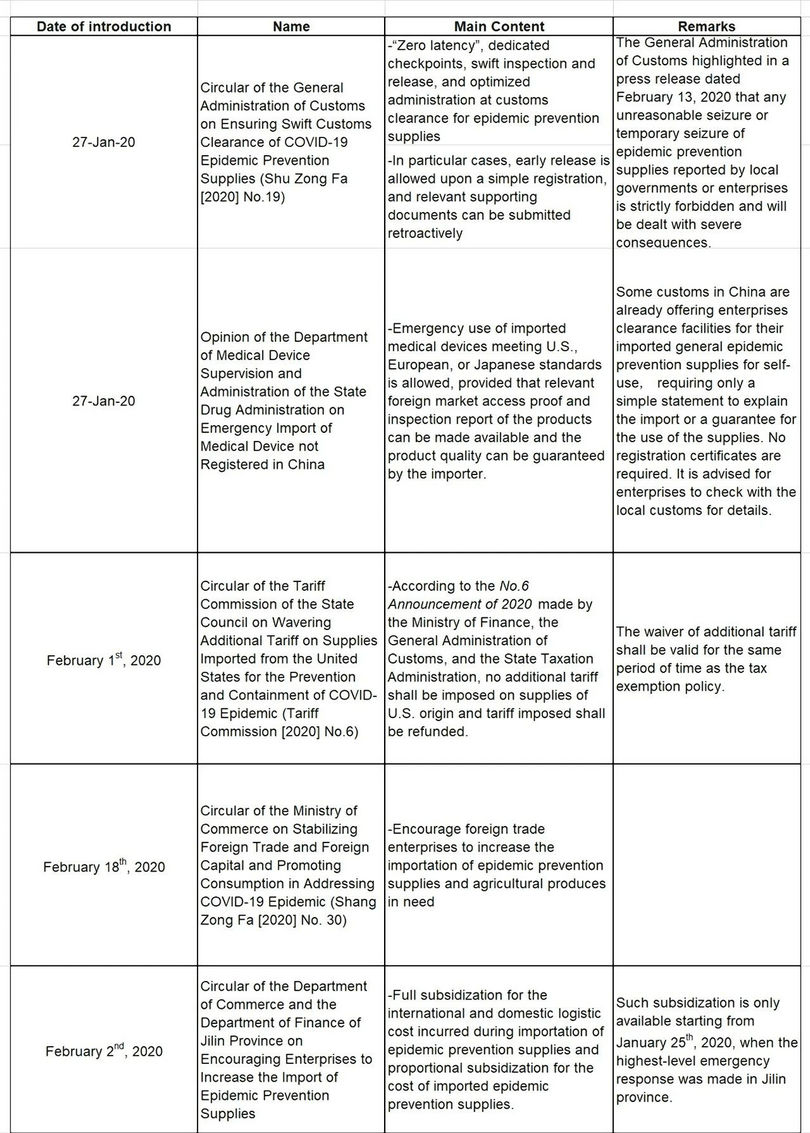
The General Administration of Customs, the Department of Medical Device Supervision and Administration of the State Drug Administration, local governments, and customs introduced a series of emergency policies for importation of prevention supplies during the epidemic. Due to limited length, only national and major local facilities are listed as follows:
V.Legal Risk Control for Epidemic Prevention Supply Import
1.Points of Attention when Signing Contracts
a.Choosing the Right Country to Import
Countries like India, Russia, Thailand, and Korea are imposing restrictions on epidemic prevention supplies like facemasks during the epidemic. It is advised to familiarize yourself with relevant regulations in countries you intend to import from and confirm if the country in question is planning to impose or have already imposed restrictions on epidemic prevention supplies. In addition, it is advised to stipulate explicitly in the contract the potential risk and obligations induced by import and export restrictions.
b.Subject Qualification Verification
As it is common for the parties to the import contract to negotiate by email or by phone call, it is advised for the importer to ask its foreign supplier to provide its subject qualification proof and credit certificate, as well as verify through internet or seek a third party to verify its subject qualification to enter into contract and capability to conclude it.
c.Describe Cargo Information in Detail
In order to ensure a smooth contract fulfillment, it is advised to fill the cargo information into the contract, including the name, specification, shipping mark, quantity, unit price, weight, value and so on. Meanwhile, considering the special nature of the medical prevention supplies, it is advised to describe the registration information and license information in detail, and include relevant registration certificates as appendices to the contract. In addition, since there might be cases where supplies will be detained or returned should it fail the customs’ spot inspection in terms of technical standards, it is advised to stipulate in the contract the obligations induced therefrom.
d.Set Payment Arrangements to Your Advantage
It is advised to make low-risk payment arrangements, such as using a letter of credit. Direct transfer of money or e-transfers should be avoided. Moreover, you should opt for a payment schedule that allows for payment after examination of the goods upon receival.
e.Set Insurance Arrangements to Your Advantage
In order to protect the importers’ interest, it is advised to choose an insurer with easy access, comprehensive coverage, and loose claim settlement policies to insure corresponding risks.
f.Choose Suitable Shipping Methods
Epidemic prevention supplies may be imported by ship, by air or by rail; it is advised to take into consideration the cost, volume, and timeliness in order to make an informed decision. In addition, some countries have rescheduled their freight to and from China since the outbreak of the epidemic. Hence, a feasibility check is highly recommended before making the choice, so as to avoid wasting time and money on communications.
g.Contract Termination / Rescission and Claim / Compensation Settlement
The uncertainty of contract fulfillment might increase due to the epidemic. It is advised for enterprises to make an informed evaluation on contract rescission and the consequent liability, and stipulate accordingly in the contract to avoid any material loss.
h.Pick the Right Governing Laws for Dispute Resolution
Arbitration is often the way to resolve disputes in international contracts. In order to reduce relevant cost and protect the interest of Chinese enterprises, using Chinese governing laws and holding arbitration in domestic institutions will be in your best interest.
2.Points of Attention in Contract Fulfillment
a.Contract Fulfillment Monitoring
It is crucial to constantly stay informed on the supplier’s contract fulfillment progress. Be thoroughly aware of the preparation, embarkation, and shipping process of the cargo, and prompt the seller whenever necessary to fulfill its obligations under the contract in order to ensure delivery on time.
b.Truthful Declaration of the Purpose of the Cargo
The purpose of the epidemic prevention supplies shall be declared truthfully at customs clearance, and the statement cannot be retracted. For example, self-use supplies declared cannot be sold for profit under any circumstances. Otherwise, administrative sanctions may be imposed by the regulators, and in severe cases it may even constitute smuggling.
c.Supplementary Clearance and Formalities
In the event that the import takes the express track at customs or other administrative departments and enjoys the convenience of priority customs clearance, enterprises are advised to check with the administrative staff for guidance to cover afterwards the declaration and clearance formalities and submit required certificates or proofs retroactively.
d.Foreign Exchange Facilities
The State Administration of Foreign Exchange issued on January 27th, 2020 the Circular on Establishing a Foreign Exchange Express Track to Support Prevention and Containment of COVID-19 Epidemic, requiring banks to streamline the purchase and payment of foreign exchange for import and increase handling efficiency. On subnational levels, many banks also introduced facilities for import-based payment of foreign exchange. Enterprises are advised to follow relevant bank policies and use the most convenient, swift, and low-cost banks to facilitate the procurement.
It has become an obvious choice for enterprises invested in epidemic prevention supply production and operation to opt for import as an additional channel to procure supplies as production in a scenario where the domestic supply is low and regulations are tightened. Before the attempt, a qualification check is recommended for enterprises that are planning to cross over so as to avoid violation. Unlicensed production or sales of epidemic prevention supplies may result in administrative sanctions and even constitute crime in severe cases. Enterprises planning to import epidemic prevention supplies should pay attention to risk prevention in contracting and contract fulfillment in order to avoid any unnecessary loss.
Authors:
Xu Yu, Partner in cross-border investment and finance practice of the Shenzhen office.
Email:sz_xuyu@dehenglaw.com
Wang Meiling
Lawyer Assistant
Wang Meiling, Trainee lawyer in cross-border investment and finance practice of the Shenzhen office.
Email:wangmeiling@dehenglaw.com
This article was written by the lawyer of DeHeng Law Offices. It represents only the opinions of the authors and should not in any way be considered as formal legal opinions or advice given by DeHeng Law Offices or its lawyers. If any part of these articles is reproduced or quoted, please indicate the source.
